Revolutionary HIV Prevention Method: Lenacapavir Hits the U.S. Market
Approval Granted for Innovative Approach to HIV Prevention in U.S. - Approval granted for innovative HIV prevention medication in the U.S.
Whoohoo! Big news in the world of HIV prevention! 🚀
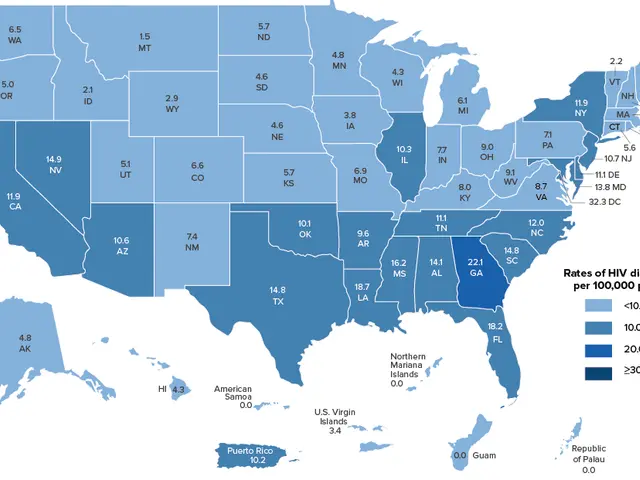
The FDA just gave a green light to Lenacapavir, a groundbreaking drug for those at high risk of contracting HIV. 💉💰
In two massive clinical trials with over 4,000 participants, this bad boy showed a whopping 99.9% effectiveness rate, making it practically a vaccine. 🦠🔥
Although it might make your wallet cringe with a $28,000 (around €24,000) price tag per person per year, experts are saying its production costs are merely $40. 💸💸💸
Oh, and there were side effects too – reactions at the injection site, sunny days giving you headaches, and a dash of nausea to keep it interesting. 🤮
So, Lenacapavir is expensive, but it's powerful! The question is: who gets to enjoy this powerhouse?
Gilead, the smart cookies who invented it, have been working on licensing deals with six generic manufacturers to bring affordable versions to low-income countries 🌍🤝. On the flip side, critics moan that millions infected with HIV live in countries where an affordable version of Lenacapavir is like a red moose in a brown field – rare, my friend!