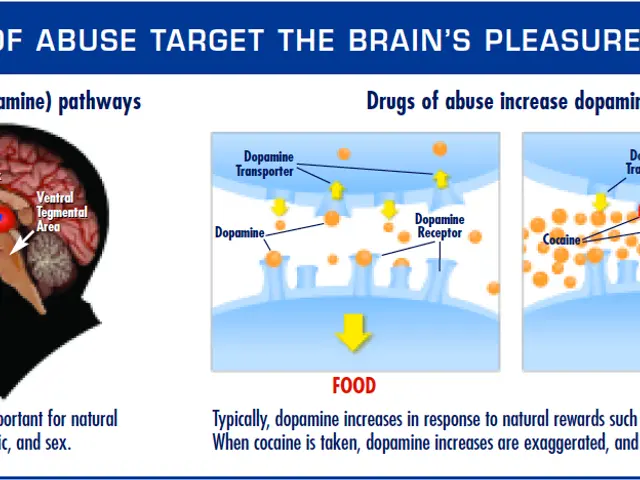
Biotech companies focused on anti-aging take off amidst a fiery competition of $101 million, propelling a worldwide rivalry against the lingering issue of aging populations.
In the realm of medical research, significant strides have been made in the development of cell-based therapies and gene therapy trials. Here are some of the latest updates:
Solid Biosciences Inc. and ProMIS Neurosciences Advance in Gene Therapy Trials
Solid Biosciences Inc. (NASDAQ: SLDB) has made progress in its Phase 1/2 INSPIRE DUCHENNE trial for SGT-003 gene therapy targeting Duchenne muscular dystrophy. The company has dosed 15 participants, with the treatment continuing to be well-tolerated and no treatment emergent serious adverse events observed.
Meanwhile, ProMIS Neurosciences has received approval from the Data and Safety Monitoring Board to advance to the final dose escalation cohort in its Phase 1b PRECISE-AD trial. No cases of amyloid-related imaging abnormalities have been observed across enrolled patients.
Fast Track Designations and Regulatory Meetings
PMN310, a potential treatment, has received FDA Fast Track designation. The FDA will meet with Solid in Q4 2025 to discuss regulatory pathways for SGT-003.
uniQure's AMT-191 has also received Orphan Drug and Fast Track designation from the FDA.
Joint Ventures and Collaborations
Avant Technologies, Inc. (OTCQB: AVAI) has formed a 50/50 joint venture with Austrianova called Klothonova, Inc., focusing on cell-based therapies utilizing encapsulated Klotho-producing cells. This venture leverages Avant's resources and Austrianova's proprietary cell encapsulation technology.
HCW Biologics Inc. (NASDAQ: HCWB) has designated one of its proprietary TRBC-pembrolizumab-based immune checkpoint inhibitors, HCW11-040, as its franchise immunotherapeutic for internal clinical development targeting solid tumors.
The Role of Klotho Protein
Klotho protein, often referred to as a "longevity protein," modulates aging processes and affects brain, heart, kidneys, and immune function simultaneously. Higher Klotho levels have been associated with up to 30% increased lifespan, while lower levels are associated with 31% higher mortality rates.
Interestingly, Klotho levels drop by 50% after age 40, creating substantial therapeutic opportunities for intervention.
Market Projections and Clinical Trials
The global Alzheimer's disease market is projected to reach $32.8 billion by 2033, while cardiovascular disease remains the world's leading cause of death, and kidney disease affects 850 million people worldwide.
The PRECISE-AD trial, designed to enroll 128 patients, is on track to report final 12-month top-line results in Q4 2026.
Solid has executed over 25 agreements or licenses for the use of its proprietary AAV-SLB101 capsid technology.
These developments underscore the promising future of cell-based therapies and gene therapy trials in addressing various health conditions, from muscular dystrophy to Alzheimer's disease and kidney disease.
Read also:
- Europe's mandatory vaccination programs advocated by health officials in the face of mounting disinformation
- Rural farm communities sound the alarm over the perilous state of livestock deliveries
- Initial Nutrient for Boosting Immune System: Reasoning Behind Blueberries Being an Ideal First Food for Infants
- EU's ban on bean exports from Nigeria results in an annual loss of $363 million for the country, according to AAPN.