COVID-19 Vaccination Update: Insights on the Novavax Vaccine
FDA Authorizes Novavax COVID-19 Vaccine for Emergency Use
The Food and Drug Administration (FDA) has approved the Novavax COVID-19 vaccine for emergency use in the United States. This decision comes after a clinical trial with over 25,000 participants in the US and Mexico, which showed an efficacy of 90.4% against mild, moderate, or severe COVID-19.
The Novavax vaccine is a protein-based vaccine, similar to the current hepatitis B and acellular pertussis vaccines. It delivers a piece of the coronavirus' spike protein to the cells to train the immune system to recognize the virus. The vaccine contains a chemical adjuvant called Matrix-M, which boosts the immune response to the protein.
It's important to note that the Novavax study was conducted before the emergence of the Delta and Omicron variants. As a result, the vaccine's effectiveness against these symptoms is expected to be lower. The mRNA vaccines, such as those made by Pfizer-BioNTech and Moderna, offer less protection against infection caused by Omicron, but still provide strong protection against severe disease and hospitalization.
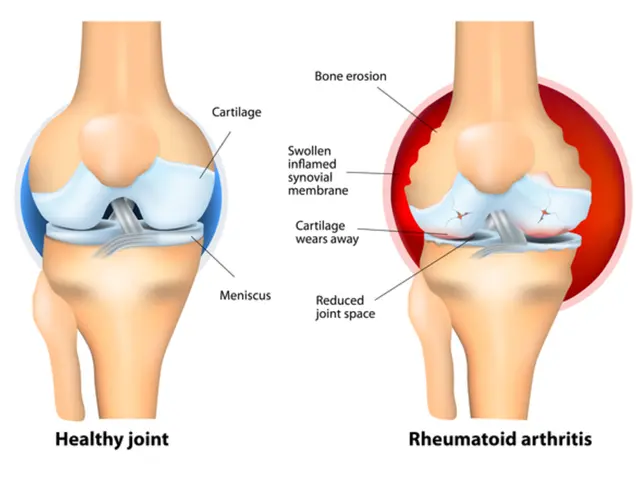
The FDA will include a warning about the increased risks of myocarditis and pericarditis in the fact sheets for the Novavax vaccine. This side effect, which is inflammation of the heart and its lining, was found to be more common in the trial, particularly in adolescent males and young men. People who experience chest pain, shortness of breath, or feelings of having a fast-beating, fluttering, or pounding heart within 10 days after vaccination should seek medical attention immediately.
The FDA has authorized the Novavax vaccine for the initial two doses, separated by three weeks. However, the agency will need to separately evaluate the use of this vaccine as a booster.
Unvaccinated people are six times more likely to die of COVID-19 compared to people who are vaccinated with at least two doses, according to CDC data. It's crucial to consider the benefits and risks of vaccination in making an informed decision about getting vaccinated.
The most common side effects of the Novavax vaccine are similar to those seen with other COVID-19 vaccines, including pain, fatigue, muscle pain, headache, joint pain, nausea, and fever. As always, if you have any concerns or questions about the Novavax COVID-19 vaccine, it's best to consult with a healthcare provider.
Read also:
- Toxic Shock Syndrome: Signs, Origins, Tampon Connection, and Further Details
- Identifying Sinus Infection Type: Discerning between Viral and Bacterial Sinusitis
- Exploring Colostrum's Storage Duration at Ambient Conditions: A Detailed Handbook
- Bishop expresses that Respect Life Month holds additional significance during the Jubilee Year