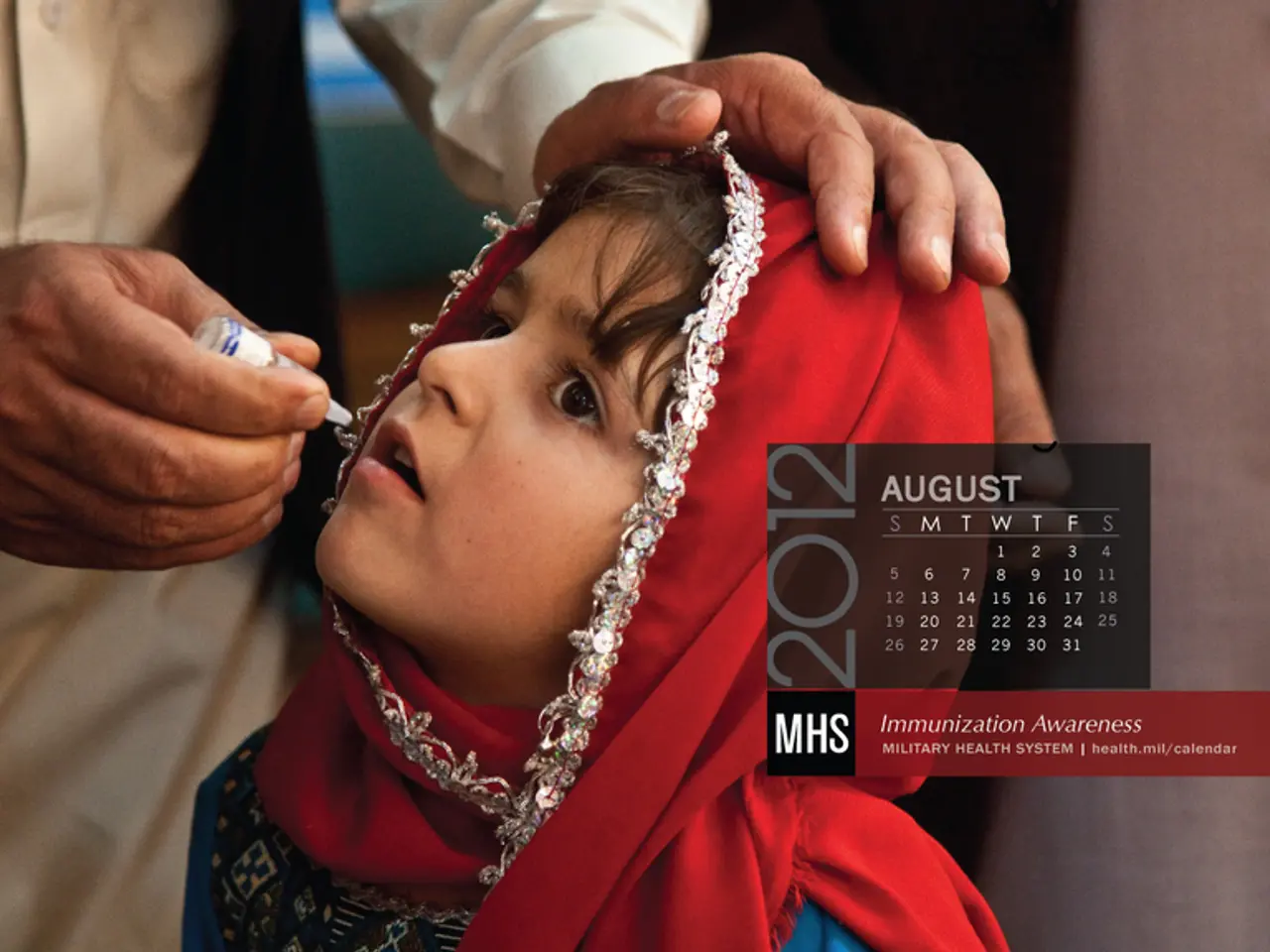
Dreyer defends Spahn amidst backlash following Astrazeneca's halt
In recent weeks, Germany has halted or restricted the use of the AstraZeneca COVID-19 vaccine due to concerns about rare cases of blood clotting events observed after vaccination, particularly in younger individuals [1][2]. This decision was not made lightly and reflects a broader European effort to maintain vaccine safety while continuing COVID-19 immunisation programs.
At the heart of this investigation is the European Medicines Agency (EMA), the European Union's regulatory body for medicines, including vaccines. The EMA has been conducting thorough reviews of the available safety data on the AstraZeneca vaccine to assess whether the blood clotting events are causally linked to the vaccine [5]. The agency has evaluated adverse event reports from multiple countries, provided updated guidance to healthcare professionals, and informed national authorities about the benefits and risks.
The role of the EMA is crucial in this situation. They aim to ensure that any decisions on vaccine use are based on scientific evidence and balance the benefit of vaccination against the rare but serious adverse effects. This collaborative investigation is aimed at providing clarity regarding the AstraZeneca vaccinations once the investigation is complete.
In Germany, the Paul-Ehrlich-Institute is the body responsible for approving vaccines. They advised the Federal Health Minister, Jens Spahn, to suspend vaccinations and re-examine them. The Health Minister would have needed very good arguments to ignore this recommendation [3].
The Rhineland-Palatinate Minister-President, Malu Dreyer, has defended Jens Spahn against criticism of the halt to AstraZeneca vaccinations. She stated that this is not the time for finger-pointing, but rather for a collaborative effort to ensure the safety of the vaccines [4].
This approach reflects a broader European effort to maintain vaccine safety while continuing COVID-19 immunisation programs. The EMA's rigorous investigation and the collaboration between national authorities and regulatory bodies are key to ensuring the safety and efficacy of vaccines in the fight against the pandemic.
[1] BBC News - Germany to limit use of AstraZeneca Covid-19 vaccine [2] Reuters - Germany restricts AstraZeneca vaccine for under-60s after blood clots [3] Deutsche Welle - German health minister under fire for AstraZeneca vaccine decision [4] Deutsche Welle - Rhineland-Palatinate Minister-President defends Jens Spahn over AstraZeneca vaccine decision [5] European Medicines Agency - Safety update: AstraZeneca's COVID-19 Vaccine Vaxzevria
- The European Medicines Agency (EMA) is conducting thorough reviews of the available safety data on the AstraZeneca vaccine to assess whether the observed blood clotting events are causally linked to the vaccine, with the aim to ensure that any decisions on vaccine use are based on scientific evidence and balance the benefit of vaccination against the rare but serious adverse effects.
- In the realm of health-and-wellness, therapies-and-treatments, medical-conditions, and science, the collaboration between national authorities and regulatory bodies, such as the EMA and the Paul-Ehrlich-Institute, plays a pivotal role in maintaining vaccine safety while continuing COVID-19 immunisation programs, providing clarity and establishing trust for the public.