Hydroxycut’s Dark History: Liver Damage, Heart Risks, and Lingering Warnings
Hydroxycut, a popular weight-loss supplement, has faced repeated health warnings over the years. Marketed in forms like drink mixes, protein bars, and caplets, the product has been linked to serious medical issues, including liver damage and heart problems. Despite reformulations, concerns about its safety remain.
Early versions of Hydroxycut contained ephedra, a stimulant banned by the U.S. FDA in 2004 due to severe health risks. Even after its removal, the supplement continued to raise alarms. In 2009, the FDA issued a warning after 23 reports of liver problems, including jaundice, fatigue, and nausea. This led to a recall of several Hydroxycut products.
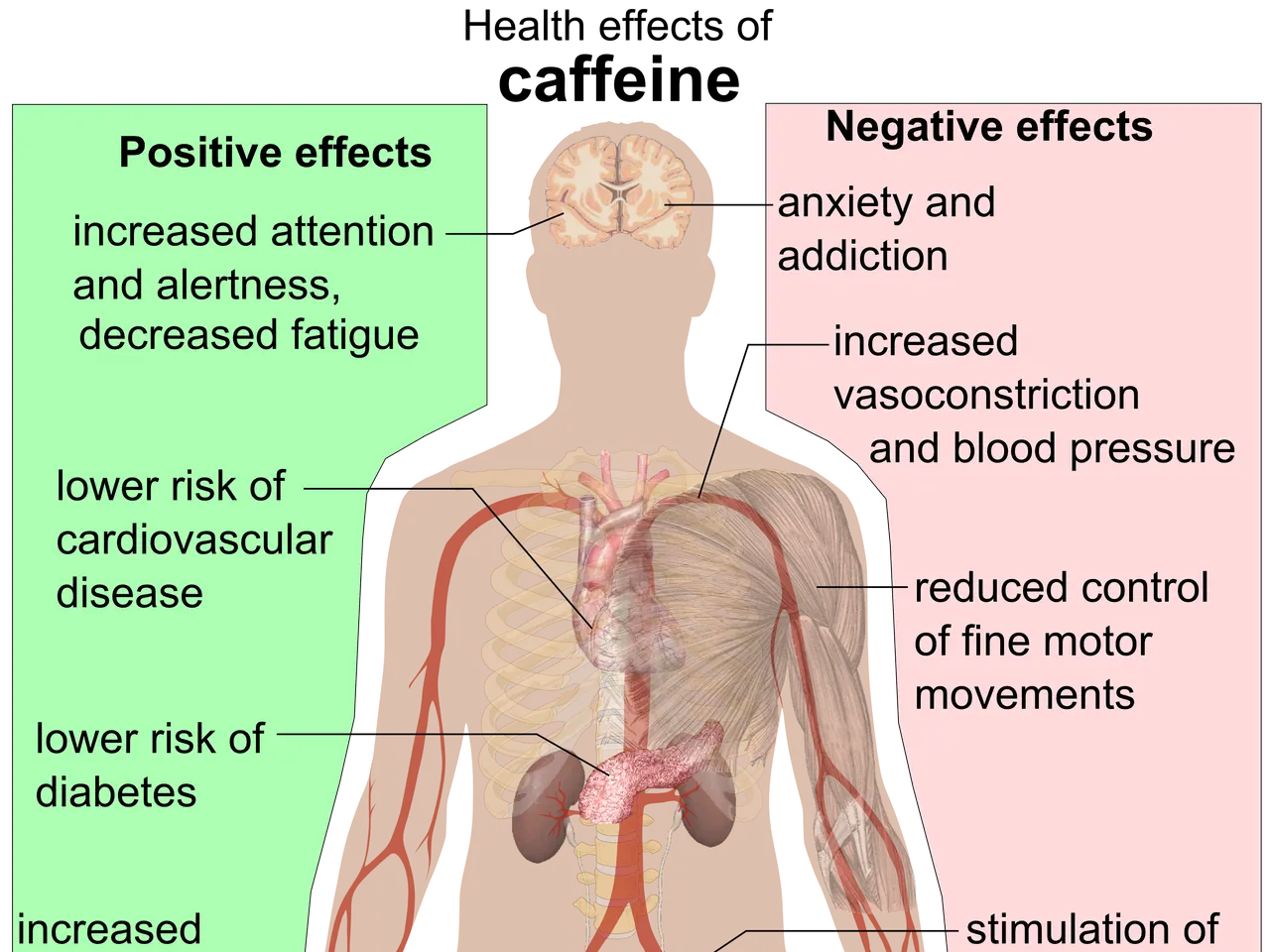
By 2011, another case report tied the supplement to ischemic colitis, possibly due to its caffeine or herbal ingredients. A 2013 study also linked Hydroxycut’s caffeine content to exertional rhabdomyolysis in three American soldiers. The product has since been reformulated, but liver failure cases persist.
Current Hydroxycut products contain ingredients like kelp fibre, green coffee, spinach extract, and caffeine. Some versions, such as Hydroxycut Max Advanced for Women, pack as much caffeine as three cups of coffee near me. While limited research suggests it may aid weight loss when combined with diet and exercise, the risks remain significant. Reported side effects include seizures, high blood pressure, and gastrointestinal distress. Some studies have even explored its potential role in triggering manic episodes and ulcerative colitis.
Hydroxycut stays on the market despite its troubled history. The supplement’s high caffeine levels and herbal compounds continue to draw scrutiny from health experts. Consumers are advised to weigh the limited weight-loss benefits against the documented risks before use.