Tragic setback in gene therapy advancements: A fatal incident that halted progress for a decade, on September 17, 1999.
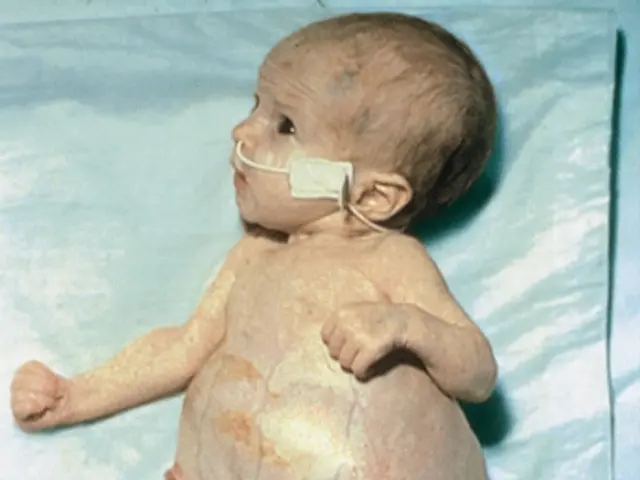
In the year 1999, the world of gene therapy faced a significant setback when an 18-year-old named Jesse Gelsinger lost his life due to a gene therapy experiment at the University of Pennsylvania. Jesse, who had a rare genetic disorder called ornithine transcarbamylase (OTC) deficiency, was participating in a trial aimed at correcting the defective OTC gene using a weakened adenovirus. Despite maintaining his health through a strict diet and medication regimen, he developed flu-like symptoms and later experienced jaundice, a severe inflammatory reaction, and a blood clotting disorder. His organs failed, and he passed away on September 17, 1999.
The tragedy led to a series of changes in the way gene therapy clinical trials are conducted. Stricter informed-consent requirements and greater monitoring for gene therapy trials became the norm. The FDA also started requiring more rigorous oversight, a response directly attributed to Jesse's death.
The field of gene therapy, which had stalled after Gelsinger's death, rebounded with progress in understanding viral vectors and the advent of CRISPR. However, it's important to note that the first FDA-approved treatment for correcting OTC deficiency was developed posthumously, although specific details about its location of development are not readily available.
The gene therapy trial that Jesse was part of was led by Dr. James Wilson, who owned stock in the company developing the therapy and stood to gain millions if it was successful. This conflict of interest raised concerns, and the FDA investigation found numerous issues with Jesse's enrollment in the trial, including poor liver function, undisclosed risks, and conflicts of interest.
Dr. Kathryn Zoon, then-director of the FDA's Center for Biologics Evaluation and Research, expressed her concerns about the deviations found in the trial. The University of Pennsylvania halted all gene therapy trials underway at the time following Gelsinger's death.
In more recent times, gene therapy has made significant strides, with scientists using it to treat many rare genetic disorders, including severe combined immune deficiency, multiple forms of blindness, and OTC deficiency. The number of approved gene therapy products is still small, with many therapies using cells that are edited in the lab and returned to the body to fight or treat cancer, rather than changing the genes in the nucleus of a patient's own cells.
The first CRISPR-based gene therapy, which treats sickle cell anemia, was approved in January 2024. This milestone marks a new era in gene therapy, one that promises to bring hope to countless individuals suffering from genetic disorders. Yet, it's a reminder of the tragic events that led to this progress, events that serve as a cautionary tale in the field of medical research.
Paul Gelsinger, Jesse's father, launched a wrongful-death suit against parties involved in the trial, which was eventually settled for an undisclosed sum. The loss of Jesse Gelsinger remains a poignant reminder of the ethical complexities and risks associated with gene therapy, and the need for continued vigilance and transparency in the pursuit of scientific advancement.
Read also:
- Rapid action required: Scientists urgently working to freeze a severely endangered tree species to prevent its extinction
- New York City Council Proposes Legislation to Eliminate Fluoride from Public Water Supply
- Impact of Alcohol Consumption During Pregnancy: Consequences and Further Details
- The cause behind increased urination after alcohol consumption is explained here.